What are the nAChRs and why are they important?
The nicotinic acetylcholine receptors (nAChRs) are the evolutionary ancient allosteric membrane proteins mediating the synaptic transmission [1]. These prototypic members of the near-ubiquitous superfamily of pentameric ligand-gated ion-channels are involved in many physiological processes (from learning to motor control), neurological diseases (Alzheimer’s and Parkinson’s diseases, schizophrenia, epilepsy), and addictions (alcohol, tobacco). NAChRs convert the chemical signal of acetylcholine into ion current by allowing sodium and calcium ions to enter the cell and potassium ions to exit the cell. This fast conversion of chemical signal to ion current relies heavily on allosteric interactions, which link the agonist-binding pocket in the extracellular domain to the gate lying approximately 60 Å away in a central channel spanning the entire length of the molecule. Hundreds of nAChR-binding compounds have been found to modulate nerve impulse transmission by regulating this coupling of agonist binding and channel gating [2][3]. The remarkable diversity of nAChR protein family is represented by about 17 subunits/genes of various types (alpha (α1 to α10), beta (β1 to β4), gamma (γ), delta (δ), epsilon (ɛ))[4], yielding around 28 known pentameric nAChR receptor types with different stoichiometries (e.g. αβδαγ (neuromuscular), α4β2), each of which possesses its own distinct characteristic pharmacological and pathophysiological properties [5].
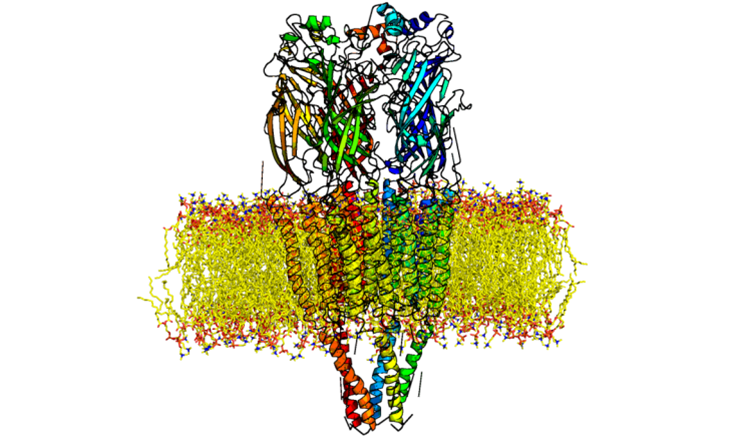
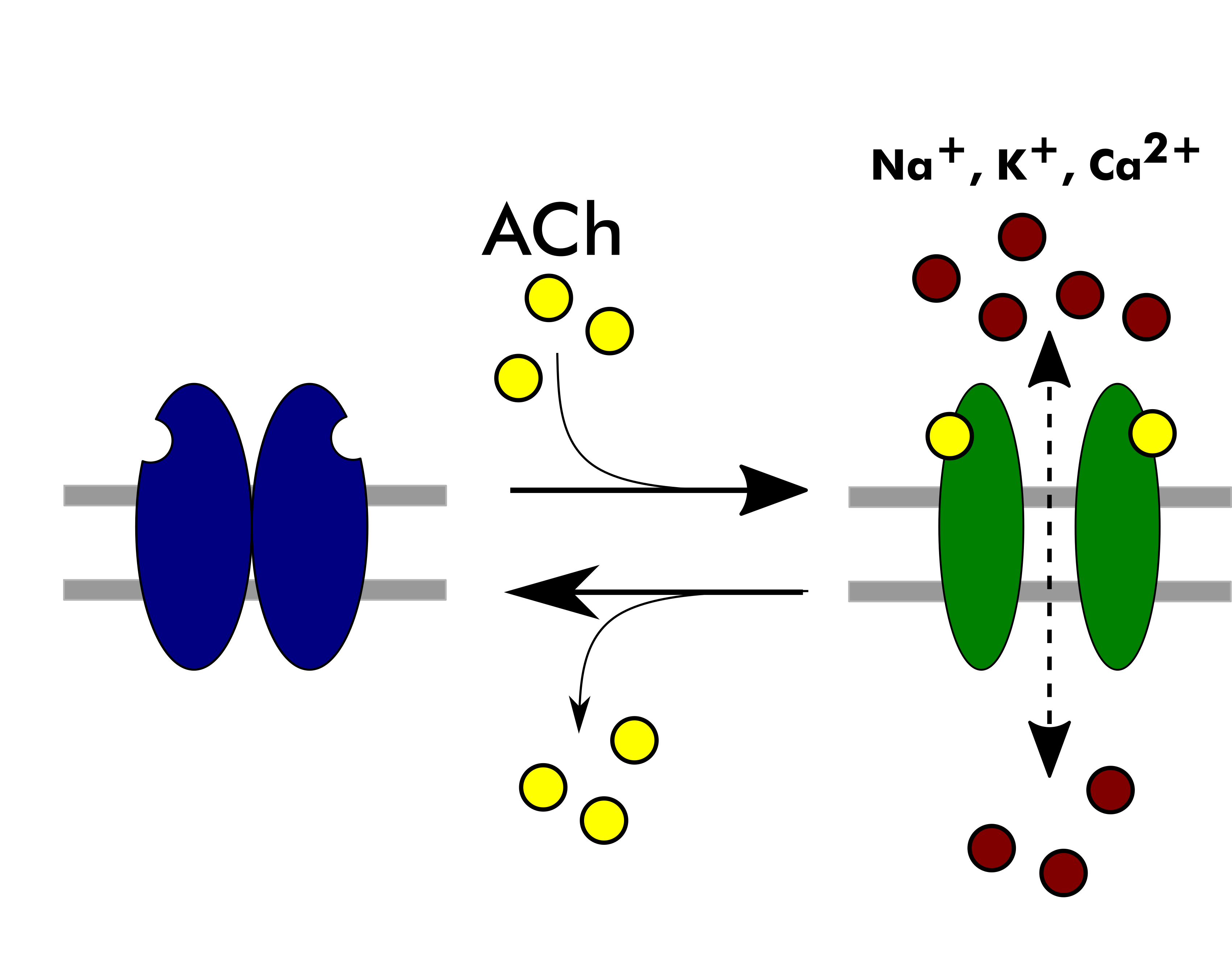
What are the current challenges?
Not surprisingly, nAChRs have remained the focus of intensive research for more than 50 years [6] since its biochemical isolation in 1970 [7]. The effort to reveal the structure-function relationships in nAChRs has resulted in huge amounts of structural and functional information. However, the cumulative knowledge on nAChRs is not systematically accessible, with thousands upon thousands of experimental and/or computational studies reporting data on various receptor types, using diverse residue numbering schemes, and different terminology. Furthermore, because nAChRs are very large and difficult to crystalize, structural studies have focused on individual parts of the molecule, which makes it challenging to compile the current knowledge and apply it efficiently to promote further discoveries [8][9]. Finally, in the absence of comprehensive and unified structural annotation, it is almost impossible to identify gaps in knowledge or areas of controversy.
How the NAChRDB can help you?
In a nutshell, NAChRDB serves as an easy way to quickly grasp the essential information regarding the functionality of important nAChR residues and regions in their structural context, at the same time providing the reference to relevant literature for detailed examination and further analysis. Broadly speaking, NAChRDB can help the researchers in nearly all the stages of the research process, from identification of gaps in current knowledge (case study #1) or the potential areas of ambiguity or controversy (case study #3), to data analysis and drawing conclusion. NAChRDB not only provides an intuitive and fast access to relevant structural-functional data on nAChRs, but also facilitates its interpretation by integrating the residue-level annotations with interactive and highly responsive visualization of sequence and 3D structure. Besides, NAChRDB can provide the users with a prediction of residues potentially relevant for the allosteric regulation of nAChRs, based on the analysis of partial atomic charges and channel lining profiles (case study #2). Feel free to check out our interactive tutorial guide and case studies to get a better idea on how NAChRDB can be used in your own research. Also, you can read more about NAChRDB in our ACS Omega article.
References
- Changeux,J.-P. (2018) The nicotinic acetylcholine receptor: a typical ‘allosteric machine’. Philos. Trans. R. Soc. Lond. B. Biol. Sci., 373.
- Gündisch,D. and Eibl,C. (2011) Nicotinic acetylcholine receptor ligands, a patent review (2006-2011). Expert Opin. Ther. Pat., 21, 1867–96.
- Reyes-Parada,M. and Iturriaga-Vasquez,P. (2016) The development of novel polypharmacological agents targeting the multiple binding sites of nicotinic acetylcholine receptors. Expert Opin. Drug Discov., 11, 969–81.
- Kalamida,D. et al. (2007) Muscle and neuronal nicotinic acetylcholine receptors. FEBS J., 274, 3799–3845.
- Millar,N.S. and Gotti,C. (2009) Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology, 56, 237–246.
- Brown,D.A. (2019) Acetylcholine and cholinergic receptors. Brain Neurosci. Adv., 3, 239821281882050.
- Changeux,J.P. et al. (1970) Use of a snake venom toxin to characterize the cholinergic receptor protein. Proc. Natl. Acad. Sci. U. S. A., 67, 1241–7.
- Changeux,J.-P. (2012) The nicotinic acetylcholine receptor: the founding father of the pentameric ligand-gated ion channel superfamily. J. Biol. Chem., 287, 40207–15.
- Halliwell,R.F. (2007) A short history of the rise of the molecular pharmacology of ionotropic drug receptors. Trends Pharmacol. Sci., 28, 214–9.